|
NANO- BIOTECHNOLOGIES |
"Innovating polymers in the biomedical arena"Dr.Rer.Nat. Theodora Krasia-Christoforou (UCY) is a Professor and the Head of the Polymer synthesis/Polymer characterization and processing Laboratories at the Department of Mechanical and Manufacturing Engineering, University of Cyprus. She received a B.Sc. in Chemistry from the University of Cyprus, (1999), a M.Sc. in Chemical Research and Diploma of the Imperial College (DIC) from the Imperial College of Science, Technology and Medicine, University of London in the U.K. (2000) and a Dr.Rer.Nat. in Physical Chemistry from the Max-Planck Institute of Colloids and Interfaces (University of Potsdam) in Germany (2003). Her research activities in the field of Materials Science and Engineering are focused on the synthesis and characterization of functional organic polymers of various chemical structures and architectures. She is particularly interested in the synthesis, characterization and applications (including biomedical) of polymer-based materials, spanning from the macroscale to the nanoscale. Several postgraduate students and more than 50 diploma thesis students working in Dr. Krasia-Christoforou’s research group have been involved in topics related to the synthesis, characterization and applications of polymer-based biomaterials. She has published 75 manuscripts/review papers in peer-reviewed scientific journals and 11 book chapters (9 Invited) on the fabrication, characterization and applications of functional, polymer-based electrospun fibrous nanocomposites and on related areas. She serves as a Reviewer for ~50 peer-reviewed journals. Furthermore, she secured funding on a National and European level via her participation either as a project coordinator or as a participant in more than 20 research projects (Group website: https://www.ucy.ac.cy/polymerlab/).
Professor Theodora Krasia Head of the Polymer Synthesis and Polymer Characterization and processing Laboratories Department of Mechanical and Manufacturing Engineering, University of Cyprus
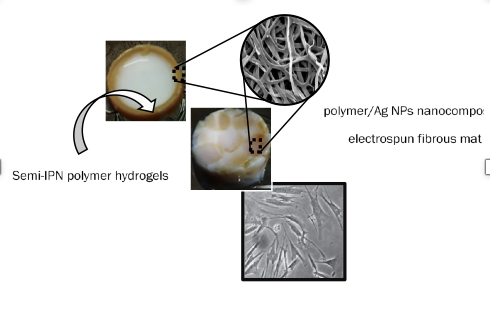
Polymer-based biomaterials (fiber/hydrogel composites)Electrospun fibers and hydrogels exhibit certain disadvantages when it comes to their use as biomaterials. More precisely, the former usually exhibit poor mechanical strength and limited directional cell growth, while electrospun nanofibrous membranes are essentially 2D and consequently they fail to mimic the 3D morphology of the Extracellular matrix (ECM). Moreover, their small pore sizes restrict cell migration and infiltration. For overcoming the above-mentioned limitations of the individual components (i.e. hydrogels and fibers), new synthetic approaches have been recently developed to combine such materials in unique, fiber/hydrogel composite structures. By integrating electrospun micro- and nanofibers within 3D hydrogels, 3D biomaterials with improved mechanical performance can be obtained, exhibiting a fiber/gel architecture, thus mimicking that of the ECM. Our group has recently synthesized chemically crosslinked polymer networks (hydrogels) within which, Ag-functionalized electrospun fibrous membranes were embedded by employing 2 different fiber dispersion modes. The resulting fiber/hydrogel composites were further investigated in respect to their swelling behavior and mechanical properties (under compression). According to the experimental data, the incorporation of electrospun fibers within the hydrogel matrix led to a significant improvement of their mechanical properties in comparison to the fiber-free hydrogel analogue. Moreover, in vitro biocompatibility studies performed using human pancreatic fibroblasts (FBs), verified the non-toxicity of the pure components (i.e. fibers, hydrogel) as well as of the combined composite structures.
|
Block copolymer-based drug nanocarriersOur work involves the synthesis and characterization of well-defined block copolymers of various chemical compositions and their further evaluation as controlled drug delivery systems in the form of block copolymer micelles. The latter consist of a hydrophilic corona and a hydrophobic core, that can host a number of hydrophobic therapeutic agents. More precisely, using controlled polymerization methods, well-defined block copolymers of various molar masses and chemical compositions can be synthesized. Via a self-assembly mechanism, such block copolymers can form micellar aggregates in solution with dimensions below 100 nm, consisting of a hydrophilic corona and a hydrophobic and/or stimuli responsive core. In the latter, a number of hydrophobic pharmaceutical compounds can be encapsulated and released under controlled conditions in a sustained manner.
Moreover, inorganic nanoparticles (for example iron oxide magnetic nanoparticles (NPs), Au NPs, etc.) may be also accommodated within the core of such nanovehicles, providing at the same time the possibility of developing new nanomaterials with potential use in theranostics. Our team has been recently involved in the synthesis of well-defined, methacrylate-based diblock copolymers by means of Reversible Addition-Fragmentation chain Transfer (RAFT) controlled radical polymerization, that were further used as nanocarriers for pirfenidone. The presence of pH responsive and aromatic functional groups within the micellar core resulted to an enhanced drug loading efficiency and promoted the controlled release of this drug under acidic conditions. The resulting pirfenidone-loaded polymeric micelles demonstrated high effectiveness in the normalization of the tumor microenvironment, thus resulting to an enhanced efficacy in terms of perfusion, immunostimulation and drug delivery.
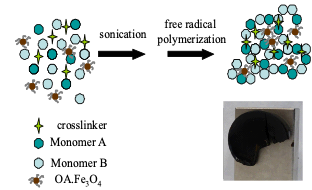
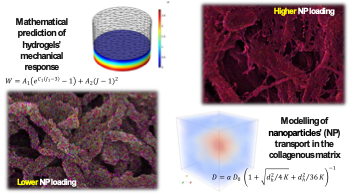
Superparamagnetic nanocomposite hydrogelsMagnetoresponsive hydrogels consisting of hydrophilic polymer networks and magnetic nanoparticles attract considerable attention, due to their potential use in various biomedical applications such as biosensors, magnetic drug delivery systems, magnetoactive tissue engineering scaffolds and bioseparation systems. Our group has been involved in the synthesis and characterization of superparamagnetic synthetic hydrogels consisting of stimuli-responsive moieties (i.e. pH and temperature responsive), combined with preformed, oleic acid-coated magnetite nanoparticles (OA.Fe3O4). Depending on the magnetic content, such materials exhibit tunable magnetization and mechanical properties. The possibility of controlling the response of such composites either by altering the temperature, the magnetic field, and the solution pH, creates new prospects in the exploitation of such materials in the biomedical field. Besides the fabrication of synthetic systems, we have been also involved in the preparation of superparamagnetic collagen-based nanocomposite hydrogels with tunable swelling, mechanical and magnetic properties. Collagen type-I hydrogels were prepared, and subsequently they were immersed in aquatic magnetic fluids containing various concentrations double-layer oleic acid-coated hydrophilic magnetite nanoparticles (OA.OA.Fe3O4). Mathematical modelling was applied to predict the mechanical response of these materials, whereas the NP diffusion and anchoring within the collagen hydrogel was also modelled.
|
Polymer-based biomaterials (fibers, hydrogels)During the last years our group emphasizes on the synthesis and characterization of polymer-based biomaterials (including polymer-based nanocomposites) in the form of electrospun micro- and nanofibers and well-defined functionalized hydrogels (physically and chemically crosslinked). Functional hydrogels We develop 3D hydrogels of variable chemical compositions, by employing RAFT controlled radical polymerization and conventional free radical polymerization. Such materials could be potentially used as drug delivery systems and tissue engineering scaffolds. Exemplarily, we have developed structure-defined hydrogels with tunable chemical composition and mechanical response. The latter can be easily modified not only by altering the chemical composition, but also via the development of semi-interpenetrating polymer networks (semi-IPN). The precisely controlled chemical composition and architecture of such materials allowed for the prediction of their mechanical response via mathematical modeling. Moreover, the in vitro biocompatibility of the produced hydrogels was demonstrated on selected cell lines.
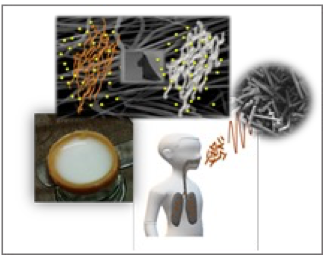
Electrospun nano- and microfibers Furthermore, we are extensively involved in the development of innovative biomaterials in the form of electrospun nano- and microfibers and well as nano- and microrods by means of electrospinning. The latter is a widely applicable method used in the production of nano- and microfibrous materials that has already reached the industrial sector. Such materials and characterized by high surface-to-volume ratios, high porosity and tremendous versatility in respect to chemical composition and morphology, that in turn govern their properties. We have developed electrospun fibers that have been evaluated among others as drug delivery systems. In addition, by applying simple, post-treatment processes, electrospun microfibers can be transformed into microrods, with potential use in inhalation medicine. |
|
SELECTED PUBLICATIONS1. Mpekris, F.; Papaphilippou, P. Ch.; Panagi, M.; Voutouri, C.; Michael, C.; Charalambous, A.; Dinev, M. M.; Katsioloudi, A.; Prokopi-Demetriades, M.; Anayiotos, A.; Cabral, H.; Krasia-Christoforou, T.; Stylianopoulos T. “Pirfenidone-loaded polymeric micelles as an effective mechanotherapeutic to potentiate immunotherapy in mouse tumor models”, ACS Nano, 2023 2023, 17, 24, 24654–24667. 2. Nikolaou, M.; Avraam, K.; Kolokithas-Ntoukas, A.; Bakandritsos, A.; Lizal, F.; Misik, O.; Maly, M.; Jedelsky, J.; Savva, I.; Balanean, F.; Krasia-Christoforou, T. “Superparamagnetic electrospun microrods for magnetically-guided pulmonary drug delivery with magnetic heating” Materials Science & Engineering C 2021, 126, 112117 (11 pages). 3. Karagiorgis, S.; Tsamis, A.; Voutouri, C.; Turcu, R.; Porav, S. A.; Socoliuc, V; Vekas, L.; Louca, M.; Stylianopoulos, T.; Vavourakis, V.; Krasia-Christoforou, T. “Engineered magnetoactive collagen hydrogels with tunable and predictable mechanical response” Materials Science & Engineering C 2020, 114, 111089 (8 pages). 4. Nikolaou, M.; Krasia-Christoforou, T. “Electrohydrodynamic methods for the development of pulmonary drug delivery systems” European Journal of Pharmaceutical Sciences, (SimInhale Special Issue) 2018, 113, 29-40. 5. Krasia-Christoforou, T.; Georgiou T., “Polymeric theranostics: Using polymer-based systems for simultaneous imaging and therapy” (feature article), Journal of Materials Chemistry B 2013, 1, 3002-3025. |