|
SYNTHETIC ORGANIC CHEMISTRY & CHEMICAL BIOLOGY LABORATORY |
“Maximizing synergies to address tomorrow’s emerging challenges in Medicine and Health”Dr Georgiades is Assist. Professor in the Department of Chemistry, UCY and Head of the Synthetic Organic Chemistry and Chemical Biology Laboratory. He has a BSc in Chemistry from UCY (2001) and a PhD in Organic Chemistry and Chemical Biology from Harvard University (2006). After postdoctoral appointments at the Scripps Research Institute - La Jolla, California (2006-2007) and Imperial College London (2007-2010), he has worked for one year as independent Lecturer in Pharmacy and Chemistry at Kingston University (UK), before joining UCY in 2011. He was promoted to Assist. Professor in 2018. His research expertise spans the areas of Organic Synthesis, Bioorganic and Medicinal Chemistry. His research interests include: (a) Development of DNA G-quadruplex ligands as new anti-cancer treatments and bio-imaging agents; (b) Synthesis and biological evaluation of small-molecule modulators of cellular signaling pathways; (c) Development of innovative catalytic technologies for C-H bond activation and medicinal applications; (d) Synthesis of self-assembled biomaterials for energy applications. Dr Georgiades serves on the Editorial Boards of Referees of many international chemistry journals and as reviewer for more than 30 scientific journals. He has been a scientific referee for proposals submitted under european funding schemes (SONATA, National Science Center, Poland; YERUN Research Mobility Awards; APVV Slovakia Research and Development Agency) and a Management Committee Member of COST Action CA15106 (CHAOS). He is a Member of the Pancyprian Union of Chemists (ΠΕΕΧ), a member of the American Chemical Society (ACS), a Member of the International Chemical Biology Society (ICBS) and a Fellow of the Higher Education Academy, UK. Assistant Professor Dr Savvas N. Georgiades Head of Synthetic Organic Chemistry and Chemical Biology Laboratory Department of Chemistry @ University of Cyprus (+357) 22892779
Conjugates of Validated anticancer drugs with optical probes, for bioimaging purposesAnother area of interest is the development of custom-designed bioimaging tools for monitoring enzymes found to be over-expressed or mutated in human cancers. One example is the Epidermal Growth Factor Receptor Tyrosine Kinase (EGFR-TK), mutations of which may result in translocation from the cell membrane to the membranes of intracellular organelles (mitochondria, nucleus), thus rendering EGFR-TK resistant to drugs. Disruptions of EGFR function often leads to aberrant cell cycle progression, proliferation, differentiation and survival. Being able to image EGFR non-invasively could aid in target detection and quantification, drug validation, dose optimization and treatment monitoring. Nonetheless, it requires the development of specialized optical probes. In this context, we have developed a protocol for conjugating EGFR-TK aminoquinazoline-type inhibitors to a Ru(bipy)3 fluorescent probe, employing a desymmetrization process for obtaining the interconnecting triethylene-glycol-derived linker. This effort has yielded an unusual theranostic agent that was successfully detected intracellularly by fluorescence microscopy. Both the fluorescence microscopy and a time-resolved FRET study indicated specific subcellular distribution in mitochondria, which was further documented by mitochondrial isolation. A theranostic of this type. |
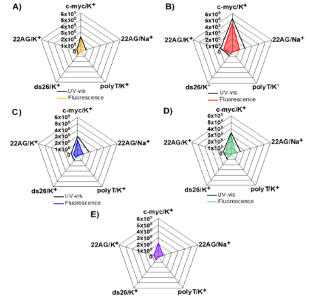
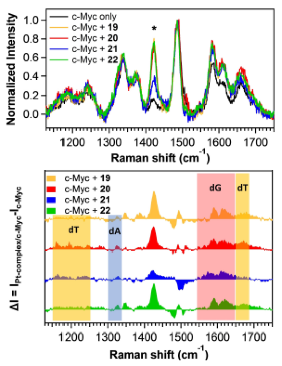
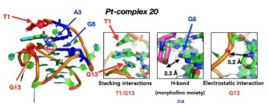
Synthesis & evaluation of Target-selective guanine-quadruplex ligands and optical probesG-quadruplexes, a family of non-canonical nucleic acid topologies, are considered emerging new targets in anticancer research, in light of their roles in inhibiting telomere elongation by telomerase, suppressing expression of known oncogenes and expression of ribosomal DNA, as well as down-regulating translation of RNAs to cancer proteins, among other functions. Small-molecule ligands of G-quadruplexes with the ability to stabilize or even drive the formation of these structures offer potential for drug discovery and unprecedented anticancer treatments, while G-quadruplex-selective binders which alter their optical properties upon binding may serve as custom diagnostic probes and sensing systems to help unravel and understand the yet unknown roles of G-quadruplexes in biological systems. Two different molecular designs have been followed by SNG team for developing innovative, selective binders and optical probes for G4s of anticancer relevance: (a) Oligoheteroaryl classes of modular ligands for G4s via 1,3-dipolar cycloadditions have generated families of modular oligomeric structures consisting of alternating pyridine and 5-membered heteroaryls (oxazoles, isooxazoles, triazoles) for targeting G4s. This molecular design addresses the issue of groove and loop interaction of G4s, since these are the loci that exhibit the highest diversity among different G4s. Rotationally flexible ligands with hydrogen-bonding ability binding in these regions are likely to enhance molecular recognition selectivity.
Applying modern synthetic tools to deliver Innovative compound libraries for high-throughput biological evaluationWe both develop and apply “cutting-edge” catalytic synthetic methodologies, often compliant with the principles of sustainable chemistry, to convert readily available building blocks to various (natural product-like or drug-like) end-products of high molecular complexity, and further derivatize them to produce new molecular libraries for biological evaluation, in the context of drug discovery. Successful examples include:
|
(b) Organoplatinum ligands for G4s, which offer obvious advantages as binders for G4s, including planar geometries for targeting terminal G-quartets, good permeation through cell membranes, enhanced stability upon cellular entry, as well as unique optical properties, allowing them to function as optical probes and bioimaging agents for visualizing intracellular G4 localization and activity. These have been enhanced by means of side-chains that mimic structural features (and binding modes) of G4-targeting peptides.
|
|
SELECTED GRANTS
|
SELECTED PUBLICATIONS
|