|
EXPERIMENTAL PHARMACOLOGY LABORATORY
|
“Translating today's discoveries into tomorrow's therapies”Dr Nikolas Dietis is an Assistant Professor of Pharmacology at the Medical School of the University of Cyprus. He teaches basic pharmacology in the MD undergraduate program and pharmacogenomics in the MSc Precision Medicine program. He is a Registered Expert at the European Commission in the field of Pharmacology (Reg: EX2022D543045), an appointed Examiner of the Cyprus State Scholarship Foundation (ID: I.K.Y.K.1.1.04.1/V). Dr Dietis graduated from the University of Portsmouth (UK) with a BSc in Pharmacology, from Nottingham Trent University (UK) with a BScHons in Neuroscience & Pharmacology, from the Natural Sciences Research Centre in Nottingham Trent University (UK) with a MRes in Applied Biosciences/Neuropharmacology and from the University of Leicester (UK) with a PhD in Pharmacology. He has previously worked for at the University of Tasmania (Australia) as a Lecturer in Pharmacological Sciences. Dr Dietis’ research interests revolve around drug discovery & drug repurposing using target-based and disease-oriented high/mid-throughput screenings in vitro and in vivo. In 2016, he founded the Experimental Pharmacology Laboratory (www.dietislab.org) at the UCY Medical School, the first drug-screening research unit in Cyprus. The lab uses modern equipment to employ various drug screening methods for the evaluation of pharmacological efficacy and toxicological profiling of novel or approved drugs against disease. In 2018 he founded the first Zebrafish Laboratory in Cyprus that employs in vivo toxicological and pharmacological assays for drug profiling. The lab is currently focusing on the use of zebrafish cancer xenograft models to study the anticancer effects of novel and approved drugs against a variety of cancers, such as glioblastoma, prostate, liver and colorectal human cancer.
Dr Nikolaos Dietis Head of the Experimental Pharmacology Laboratory Medical School, University of Cyprus
|
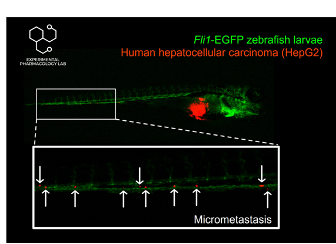
Zebrafish Cancer Xenograft Models forDrug RepurposingOur lab develops zebrafish cancer xenograft models with the microinjection of various cancer cell lines, such as glioblastoma (U87MG), liver cancer (HepG2 & Hep3B), and prostate cancer (PC3), to assess the anticancer efficacy of different drugs, with a focus on approved clinical drugs for drug repurposing. A fluorescent stereoscope is used to monitor tumor size/growth, cancer cell dissemination, angiogenesis/vascularization and metastasis, using red fluorescent-labelled injected cancer cells and a green-fluorescent transgenic zebrafish line Tg(fli1:egfp), under the presence or absence of drugs. The overall viability duration of zebrafish is used as a vertical efficacy marker in drug profiling. Orthotopic glioblastoma xenograft models are used for studying the efficacy of drugs against cancer cells through the blood brain barrier. The zebrafish's transparency and rapid development from embryo to fully-developed larvae (5 days) allow for a rapid, real-time, non-invasive observation of cancer development, making it an ultra-efficient in vivo model for anticancer drug discovery & repurposing projects.
|
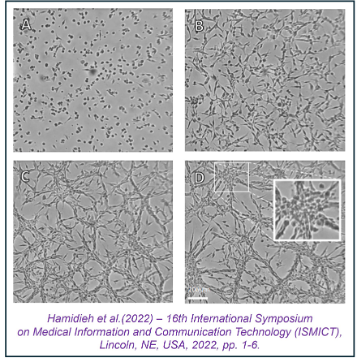
High-Throughput Screening ofApproved Drugs for Anticancer EfficacyWe utilize a comprehensive high-throughput screening (HTS) platform to evaluate the in vitro anticancer efficacy of over 800 approved clinical drugs housed in our in-house drug library. This system is optimized for rapid, large-scale drug screening, leveraging 96- to 356-well plate formats and state-of-the-art multimodal microplate readers (Tecan Spark 20M). This equipment integrates time-resolved fluorescence, luminescence, and absorbance detection to monitor cellular functions and behavior in response to drug treatments, with automated injectors for precise and efficient drug delivery. Advanced techniques such as FRET, BRET, and AlphaScreen are employed to uncover more complex molecular interactions. To support long-term cell studies, the system is equipped with integrated temperature and CO2 control modules. Pharmacological efficacy is measured using a range of indices derived from dose-response relationships, including IC50, EC50, Emax, pKa, Kd, and pA2. Beyond HTS assays, we investigate the anticancer effects of drugs using specific assays such as the scratch assay for cancer cell migration and the clonogenic assay for colony formation. Our integrative and holistic workflow enables us to work across various stages of drug discovery, from target validation and hit identification to hit-to-lead development and lead optimization, prior to in vivo preclinical screening.
|
In Vitro and In Vivo Toxicological Profiling ofNovel CompoundsWe conduct comprehensive toxicological profiling of drugs and natural compounds through a dual in vitro and in vivo toxicity/safety approach. For in vitro testing, we utilize healthy human cells (HEK or HaCat) to assess cytotoxic effects, including cell viability, metabolic activity, membrane integrity and cell proliferation, employing advanced automated equipment such as the Countess II cell viability counter. In vivo toxicological assessments are performed using zebrafish larvae as a model organism, allowing us to monitor morphological, developmental, and behavioral changes in response to drug exposure. These evaluations provide essential toxicological parameters such as Lethal Dose 50% (LD50), No Observed Adverse Effect Level (NOAEL), and Maximum Tolerated Dose (MTD). Additionally, our workflow supports detailed analysis of preclinical toxicity metrics, including BenchMark Dose 10% (BMD10) and Lowest Observed Adverse Effect Level (LOAEL) following OECD guidelines. This combined approach of in vitro and in vivo assays, integrating cytotoxicity cell-based assays with zebrafish-based live toxicity screens, enables us to assess both the acute and chronic toxicity profiles of drugs in simple and more complex environments.
|
 |
|
SELECTED PUBLICATIONS
|