|
NANOTECHNOLOGY FOR MOLECULAR IMAGING AND ANALYSIS |
“Engineering biomolecular solutions for health”Chrysafis Andreou, Ph.D. joined the Electrical and Computer Engineering department of the University of Cyprus as a lecturer in 2018 and was promoted to Assistant Professor in 2022. He is the head of the Nanotechnology Imaging and Detection Lab. He obtained two B.Sc. degrees (in Physics and in Mathematics) from the Pennsylvania State University (2006), a M.Sc. in Electrical Engineering from the University of Cyprus (2008), and a Ph.D. in Biomolecular Science and Engineering from the University of California Santa Barbara (2013). During his Ph.D. studies, he developed microfluidic systems for biomolecular detection based on optically active nanoparticles providing surface enhanced Raman scattering. Some of the most relevant applications include detection of methamphetamine in saliva, detection of antibiotics in milk, and real-time analysis of gaseous flows. He has extensive experience in nanoparticle synthesis, sample acquisition and processing, and data analysis with advanced chemometric techniques, as well as numerical simulations for chemical transport phenomena. Additionally, he worked as a Research Scholar at the Memorial Sloan Kettering Cancer Center in New York (2014-2018) where he engineered molecularly specific SERS nanoprobes for the detection of tumor-related targets in models of cancer, and specifically for applications in immunotherapy. He developed new imaging methods based on nanoparticles for Raman and photoacoustic imaging, as well as algorithms for spectral unmixing, image segmentation, and classification. Now, as a Principal Investigator, he is engineering health-related applications based on microdevices and nanoparticles, focusing on personalized medicine. Assistant Professor Chrysafis Andreou, PhD Nanotechnology, Imaging and Detection Laboratory Department of Electrical and Computer Engineering University of Cyprus
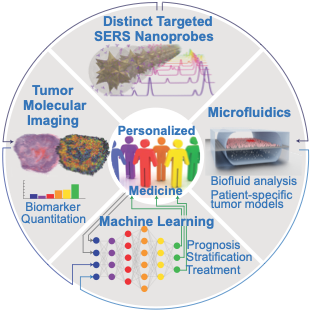
Molecular ImagingDiagnosis in full colorNanoparticles make an ideal weapon in the fight against cancer. They can be used for imaging, diagnosis, and therapy. Our lab seeks to develop new nanoparticles that use light to visualize many different targets in the tumor microenvironment at once, or to provide therapy only where needed.
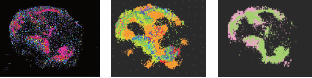
By making different dye-antibody pairs we can create a library of imaging agents, each specific to its target and spectrally distinct from the others. We used a library of SERS NPs to image tumor and immune cells and map the effects of immunotherapy. We synthesized SERS NPs able to recognize specific biomolecules related to cancer and to the body’s immune cells, and tested them in preclinical models of cancer subjected to immunotherapy. By imaging multiple molecular targets in a single scan, we were able to identify tumor cells, immune cell infiltration and activation status. This multiplexed molecular image allowed us to identify individual tumors and tumor sub-regions with possible resistance to therapy. Currently, immunotherapy benefits less than 20% of patients. We hope that imaging methods like ours can identify which patients will benefit from this treatment, or even identify newer immunotherapies for the other 80%. |
Personalized MedicineFour technologies – a single goalIn our lab, the Nanotechnology, Imaging and Detection Laboratory (NIDL), we are paving the way to Personalized Medicine. Modern medicine relies on population statistics for diagnosis and treatment, ignoring the specific circumstances, genetic factors, and molecular peculiarities of the individual. This equalitarian approach becomes especially problematic when applied to cancer, as each patient, and each tumor within each patient, have distinct cellular makeup and molecular expression – like morbid snowflakes, no two are the same. Tumor heterogeneity leads to variable treatment response, curing some patients while having little improvement for others.
At the NIDL, we develop the tools that allow us to identify molecular characteristics specific to the patient and the disease, to predict the best possible treatment. We approach this problem on four different scales, using four distinct technologies:
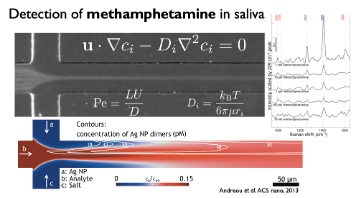
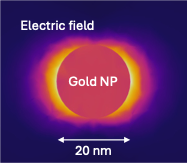
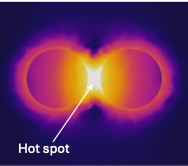
MicrofluidicsPrecision analysis fastMolecules that may cause or indicate the presence of disease can be found in our bodies, our food, and our environment. We develop molecular assays, based on nanoparticles to enrich, isolate, and detect molecules of interest. These molecular targets include antibodies against diseases (such as the coronavirus SARS-CoV2), bacterial metabolites in urine samples, acrylamide in fried and baked foods, narcotics or poisons, and many more.
We use microfluidic devices to handle minute volumes of samples. The samples processed and mixed with nanoparticles with high precision to enhance and detect their Raman signals. In this way, we can detect trace quantities of analytes with high specificity. Living model systemsMicrofluidic channels have similar sizes as blood vessels. The channels can be made to behave like biological tissues, allowing the growth of cells within them. These organ-on-chip systems allow us to study the biological processes in healthy and diseased tissues. They recapitulate the tumor microenvironment and make an excellent system for testing our nanoparticles. We envision personalized chips, populated with cancer cells extracted from each patient, to enable personalized diagnosis and treatment. |
|
|
SELECTED PUBLICATIONS1. M. Constantinou, C. Panteli, L. Potamiti, M.I. Panayiotidis, A. Agapiou, S. Christodoulou, C. Andreou, Advancing Breath-Based Diagnostics: 3D Mesh SERS Sensor via Dielectrophoretic Alignment of Solution- Processed Au Nanoparticle-Decorated TiO2 Nanowires, Advanced Sensor Research, 2024. 2. M. Stavrou, N. Phung, J. Grimm, and C. Andreou, Organ-on-Chip systems as a model for nanomedicine. Nanoscale, 2023. 3. C. Andreou, K. Plakas, N. Berisha, M. Gigoux, L.E. Rosch, R. Mirsafavi, A. Oseledchyk, S. Pal, D. Zamarin, T. Merghoub, M.R. Detty, M.F. Kircher, Multiplexed molecular imaging with surface enhanced resonance Ra- man scattering nanoprobes reveals immunotherapy response in mice via multichannel image segmentation, Nanoscale Horizons, 2022. 4. C.Andreou, R.Weissleder, M.F.Kircher, Multiplexed Imaging in Oncology, Nature Biomedical Engineering, 2022. 5. Y. Gregoriou, G. Gregoriou, V. Yilmaz, K. Kapnisis, M. Prokopi, A. Anayiotos, K. Strati, N. Dietis, A. I. Con- stantinou, C. Andreou, Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells, Nanotheranostics, 2021
|